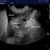
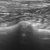
Which imaging modality is optimal for diagnosing cardiogenic pulmonary oedema and other lung pathology in dogs and cats?
Hot off the JSAP press we have just been reading the new and exciting:
J Small Anim Pract 2021 Mar;62(3):178-186
Diagnostic accuracy of a lung ultrasound protocol (Vet BLUE) for detection of pleural fluid, pneumothorax and lung pathology in dogs and cats
L Cole, M Pivetta, K Humm
https://onlinelibrary.wiley.com/doi/10.1111/jsap.13271
‘Results: When using CT as the reference standard, detection of ≥3 B lines with thoracic ultrasound had a sensitivity of 18.33% and specificity of 98.4% for detection of site specific alveolar‐interstitial syndrome. The sensitivity of Vet BLUE to detect alveolar‐interstitial syndrome increased to 56.9% when including the presence of any B line as abnormal. Overall accuracy for detection of alveolar‐interstitial syndrome based on these two criteria was 79% and 73%, respectively. Vet BLUE correctly identified consolidation in 58.3% (14/24) sites, pleural effusion in 66.6% (2/3) cases, pneumothorax in 33.3% (1/3) cases and intrathoracic mass in 25% (1/4) cases‘
‘Clinical Significance
The Vet BLUE protocol is a useful technique to detect alveolar‐interstitial syndrome and other thoracic pathology but should not be used as a sole imaging method. Detection of ≥3 B lines is highly suggestive of alveolar‐interstitial syndrome and warrants further diagnostics.’
On the face of it this is a little disappointing. We tend to feel that ultrasound is a good tool for diagnosing pneumonias and pulmonary oedema in our patient species. A sensitivity of 58% for consolidation (essentially pneumonia) is significantly below that documented in human medicine:
Pediatrics 2015 Apr;135(4):714-22.
Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis
Maria A Pereda 1, Miguel A Chavez 2, Catherine C Hooper-Miele 1, Robert H Gilman 3, Mark C Steinhoff 4, Laura E Ellington, Margaret Gross, Carrie Price, James M Tielsch, William Checkley
https://pediatrics.aappublications.org/content/135/4/714.long
In which study ‘LUS had a sensitivity of 96% and specificity of 93%’
Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis.
Chavez MA, Shams N, Ellington LE, Naithani N, Gilman RH, Steinhoff MC, Santosham M, Black RE, Price C, Gross M, Checkley W.
Respir Res. 2014 Apr 23;15(1):50
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4005846/
Demonstrating that ‘Sensitivity and specificity for the diagnosis of pneumonia using LUS were 94% and 96%, respectively‘
We are not aware of any comparable data for dogs or cats until Cole et al.
Turning to ‘interstitial syndrome’ (i.e. oedema of all causes lacking concurrent consolidation), a sensitivity of 57% when any B line was considered the diagnostic criterion also looks a little low.
Acad Emerg Med 2014 Aug;21(8):843-52
Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis
Mohammad Al Deeb, Skye Barbic, Robin Featherstone, Jerrald Dankoff, David Barbic
https://onlinelibrary.wiley.com/doi/full/10.1111/acem.12435
This meta-analysis from human medicine found that ‘The seven studies were rated as average to excellent methodologic quality. The sensitivity of US using B‐lines to diagnosis acute cardiogenic pulmonary oedema (ACPE) is 94.1% and the specificity is 92.4%‘
The authors concluded that ‘In patients with a low pre-test probability for ACPE, a negative US study can almost exclude the possibility of ACPE.’
Obviously, in all of these studies the specificity does depend somewhat on the study population and inclusion criteria.
The criteria for diagnosis of ACPE used in those studies is worth looking at since it summarises the situation nicely:
‘No restriction was made on the protocol of lung US used to diagnose ACPE, as long as the authors specified that they used B-lines to make the
diagnosis. No restriction on the type of machine or probe was applied. No restriction was placed on the type of physician performing the US as long as it was done by a non-radiologist physician at the patient’s bedside.
For the diagnosis of ACPE, or more specifically diffuse interstitial syndrome, the Volpicelli method (which divides the chest into eight zones) requires at least two zones to be positive on each side of the chest. A zone is considered positive if at least three B-lines are present in an intercostal space. Vitturi et al. used a cutoff of eight B-lines in the whole chest to be considered positive, and Gargani et al. used a comet score of more than five to represent a cardiogenic cause of acute dyspnea. Although there is not a standardized threshold for the diagnosis of ACPE, there is a linear correlation between the number of B-lines and the degree of extravascular lung water.’
At the end of the day that last line is the crux: whichever system you use, broadly, in practice, the more B lines the more chance of pulmonary oedema.
In dogs we do have some relevant published data:
J Vet Intern Med 2017 May;31(3):700-704
Assessment of Lung Ultrasound B-Lines in Dogs with Different Stages of Chronic Valvular Heart Disease
T Vezzosi 1, T Mannucci 1, A Pistoresi 1, F Toma 1, R Tognetti 1, E Zini 2 3 4, O Domenech 2, E Auriemma 2, S Citi
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5435052/
‘Dogs in stage C, with radiographic signs of PE, had numerous or confluent B‐lines in 18 of 20 cases (90%). Lung ultrasound examination detected PE with a sensitivity of 90%, specificity of 93%‘
So why the difference in diagnostic accuracy between Cole et al. and these other studies? It’s possible that non-specialist vets (with ‘basic training in emergency ultrasound’) performed less well than full-time sonographers. However, the same scenario is also the basis for the pulmonary oedema study of Al Deeb et al..
It could be that using a standardised methodology and accessing only specific sites by parting the hair (rather than clipping), as was the methodology in Cole et al., reduces sensitivity. In real life, faced with a patient with respiratory signs, one tends to search all areas of the lungs repeatedly and to extend the areas clipped until either abnormalities are found or all possibilities are exhausted.
It may be worth considering how strictly ‘B lines’ were defined and which factors might affect the number seen in this study:
J Thorac Dis. 2016 Jun; 8(6): 1356–1365.
Lung B-line artefacts and their use
Christoph F. Dietrich, Gebhard Mathis, Michael Blaivas, Giovanni Volpicelli, Armin Seibel, Daniel Wastl, Nathan S. S. Atkinson, Xin-Wu Cui, Mei Fan and Dong Yi
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4885976/
These authors list 20 factors which may influence numbers and appearance of B lines (table 1): including aspects of machine, probe, settings, patient and sonographer. Even subtle changes in settings such as focus depth and harmonics may influence B line numbers. To maximise B lines, set the focus depth at the pleural line.
For example, Figure 2 in that article demonstrates the effect of using different probes. What looks like a B line with one probe may not satisfy the usual criteria when viewed with another. Specifically, ‘short B lines’ are commonly seen with linear probes and can be difficult to categorize. As the authors of that paper say ‘many descriptions of B line artefects have been published and are contradictory‘.
There is an international consensus document in the human lung ultrasound field:
International evidence-based recommendations for point-of-care lung ultrasound.
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS).
Intensive Care Med. 2012 Apr; 38(4):577-91.
https://pubmed.ncbi.nlm.nih.gov/22392031/
And according to that, B lines must run to the bottom of the field. We presume that this was a criterion for identifying B lines in the present study.
With the benefit of 8 years of hindsight since that consensus was published I think we’d maybe be less categorical about that. There’s a great experimental, in vitro study looking at the causation of B lines:
On the influence of imaging parameters on lung ultrasound B-line artifacts, in vitro study
The Journal of the Acoustical Society of America 148, 975 (2020)
Federico Mentob, Libertario Demic
https://asa.scitation.org/doi/10.1121/10.0001797
..In which the marked effect of frequency on B line intensity is demonstrated (5-6 MHz being the frequency range causing greatest intensity).
Perhaps using CT as ‘gold standard’ is not valid. It’s certainly true, as the authors of Cole et al. state, that CT is widely ‘considered to be the reference standard for diagnosis of thoracic disease’. However, it’s worth reviewing the evidence behind this state of affairs:
European Journal of Radiology Open
Volume 7, 2020, 100273
European Journal of Radiology Open
Simple quantitative chest CT for pulmonary edema
Maria Barile, Tomoyuki Hidaac, Mark Hammerc, Hiroto Hatabua
https://www.ejropen.com/article/S2352-0477(20)30062-9/fulltext
This is a bang-up-to-date article from leading figures in the USA and Japan. It seems fair to assume that they know what they’re talking about when they say:
‘CT is believed to have a greater sensitivity for the detection of many pulmonary disease conditions when compared to conventional chest radiography. However….To our knowledge, no studies have evaluated the sensitivity of CT versus [chest x-ray] in the detection of pulmonary edema.’
It’s possible that interpretation of CT images might have falsely over-estimated the incidence of alveolar-interstitial syndrome. ‘Ground-glass’ opacity on CT was used as the criterion for diagnosis of alveolar-interstitial syndrome (AIS). However,….
‘ground-glass opacity seen on a CT image of a dog is simply considered a nonspecific finding that might be observed with various diseases or an incidental finding associated with expiration and the dependent portion of a lung lobe, particularly when clinical signs do not exist.‘
Am J Vet Res . 2017 Mar;78(3):279-288
Effect of position and time held in that position on ground-glass opacity in computed tomography images of dogs
Sang-Kwon Lee, Seungjo Park, Byunggyu Cheon, Sohyeon Moon, Sunghwa Hong, Hyun Cho, Dongwoo Chang, Jihye Choi
https://pubmed.ncbi.nlm.nih.gov/28240951/
In Cole et al., we are not told what measures were taken to exclude the possibility of artefactual ground-glass opacity (e.g. whether lateral recumbency was studiously avoided during induction and preparation). The spectrum of patients recruited to the study is inevitably skewed by the necessity to limit inclusion to only those who were subjected to CT scan. Thus, no patients with congestive heart failure are amongst the 31 patients forming the core of the study who received both lung ultrasound and thoracic CT (CHF patients were presumably filtered out at an earlier stage of patient selection at the hospital…which in itself tells you something!). 10/31 had only extra-thoracic disease. 16 had either pleural, mediastinal or airway conditions. We are left with only 5 animals with a final diagnosis of pulmonary disease (3 neoplasia, 1 ARDS, 1 PTE). Since 60 sites were reported as being ‘CT positive’ for AIS (with 8 sites being assessed per patient), a significant proportion of these must have been in patients without pulmonary disease. To an independent observer the obvious question is ‘why did those patients have AIS?’ There’s really no way to verify that AIS was really present at all of those sites. In contrast, in a study such as Vezzosi et al. looking at a relatively homogeneous population of dogs with congestive heart failure one can be pretty confident from inclusion criteria that pulmonary oedema was genuinely present in stage C dogs.
In summary, our views on all this are:
- We’re not big fans of limited thoracic ultrasound protocols in dogs. It only takes few seconds and minimal stress to clip a decent part of a dog’s chest. We suspect that thorough examination of a clipped thorax is a better plan in a dog with respiratory signs. It seems unwise to limit the examination artificially. Dyspnoeic cats do require a much more cautious, tailored approach.
- We remain of the opinion that thorough thoracic ultrasound likely has high sensitivity for consolidation/pneumonia in cats and dogs as in human patients.
- Lung ultrasound is clearly not 100% sensitive for alveolar-interstitial syndrome (pulmonary oedema of various causes). However, it’s very likely not as insensitive as this study suggests. Radiography and CT also have significant disadvantages: although we do agree that a multi-modal approach is often a good plan. In particular, one has to bear in mind the complex set of factors influencing the appearance of B lines. ‘Short B lines’ may also indicate AIS.
In real life, sonographers consider a complex algorithm involving signalment, presenting signs, physical examination, echocardiography and lung ultrasound. All of these are important in building a picture.