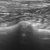
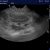
The 2024 ‘Italian Protocol’ for lung ultrasound scoring
I like this new article on lung ultrasound:
J Vet Intern Med 2024 Jan-Feb;38(1):336-345
Lung ultrasound score in dogs and cats: A reliability study
Stefano Oricco, Daniele Medico, Ilaria Tommasi, Richard Marcello Bini, Roberto Rabozzi
https://onlinelibrary.wiley.com/doi/full/10.1111/jvim.16956
Obviously, lung ultrasound has been a fast-evolving area in recent years with new ideas coming and sometimes going again! This particular proposal I like for several particular reasons.
- It allows for assessment of the whole lung which is accessible to transthoracic ultrasound (as opposed to selected points). I can see the rationale for designated points but, in practice, it doesn’t take long to examine the whole thorax and limiting oneself to a few pre-selected points will often result in missing lesional areas. Plus one often comes across a situation where in the general area of a pre-selected point one could choose, with equal validity, lung changes of different types or severity.
- The authors just make a straightforward recommendation to scan the patient in a standing or sternal position, using a linear probe aligned with the intercostal spaces (something close to transverse plane).. all of which seems sensible to me. I think it’s good practice to examine the lungs and thorax in all patients as the first step in any ultrasound exam: before restraining them in lateral recumbency. Linear probes give best definition of the pleural line and generally-speaking the aim is to examine the pleurae and lungs rather than the ribs!
- Semi-quantitative is good. I find counting B lines a bit difficult. Semi-quantitative scoring feels more appropriate. As the authors note, the human literature shows a closer correlation between total extravascular lung water (TELW) and % of length of pleural line affected with vertical artifacts than between TELW and number of B lines counted.
- Lumping the causes of ’tissue-like pattern’/complete loss of aeration makes sense. the human literature documents that, in some cases, it can be hard to distinguish reliably between different causes of this pattern (pneumonia, atelectasis, neoplasia).
- A lung score is a useful concept: it’s a meaningful indicator of severity and allows tracking progression -particularly where multiple clinicians are involved over the course of case management
OK, so what’s involved in the ‘Italian Protocol’?
They use a system of four quadrants on each side. As described, the dividing lines are drawn at at the 6th intercostal space and from the point of the elbow. In reality, trying to do this, I find that the point of the elbow is actually below the sternum in quite a few dogs! Having reported this to the author (who very kindly replied immediately), I think the general idea should be to just divide the side of the chest into 4 equal parts.

Each quadrant should be examined and lung patterns categorised:
- A-profile: a normal lung, characterized by the presence of horizontal artifacts (A-lines) that should be associated with a dynamic sign such as lung sliding to confirm a normal lung touching the chest wall, with normal to and from movement of the visceral and parietal pleural surface.
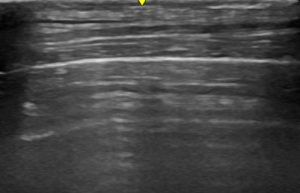
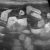
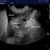
- B-profile (interstitial syndrome): at least 3 vertical artifacts (B-lines) in 1 quadrant and a decrease in aeration depending on how much these artifacts affect the pleural surface.
B profile: Vertical artifacts due to cardiogenic pulmonary oedema
- Irregular pleural line: a hypoechoic band with an irregular boundary; the pleural line can be rough or patchy, even discontinuous, during loss of aeration; it is important to differentiate it from pleural thickening, which is only present in pleural diseases such as fibrosis or chronic inflammation. Pleural irregularity results from mild peripheral loss of lung aeration, and the observed increase in thickness is not to be attributed to pleural tissue. For this reason, “irregular” is more suitable.
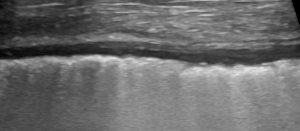
Pleural thickening in a dog with chronic pleuritis
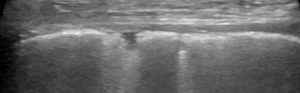
Irregular pleural line in a dog with a chronic pneumopathy
- Tissue-like pattern (alveolar syndrome): complete loss of aeration in the affected area caused by consolidation or atelectasis. Consolidation occurs when inflamed or neoplastic tissue replaces aerated tissue, whereas atelectasis is a loss of aeration without any increase in tissue. Both can be peripheral or translobar, with the former describing subpleural or focal loss of aeration.
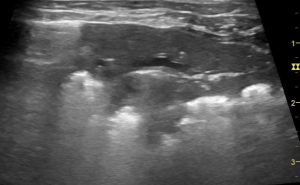
Tissue-like pattern: classic ‘shred sign’/’Fractal sign’ of non-translobar bronchopneumonia
- Pleural effusion: fluid collection in the pleural space, which could be anechoic, hypoechoic, flocculated, finely corpuscular, or anechoic with isolated fibrin branching.
Numerical scores are allocated as follows:
LUSS = 0 (A-profile) if there were horizontal artifacts (A-lines) or a maximum of 2 vertical artifacts (ie, B-lines).
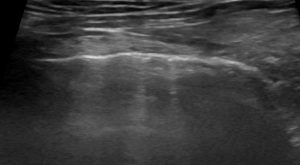

LUSS = 1 if there were vertical artifacts (B1-profile), or patterns such as pleural irregularity or slight subpleural loss of aeration that involved ≤50% of the visualized pleural line.
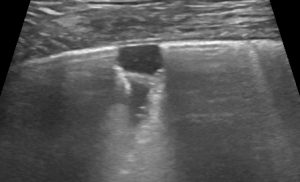
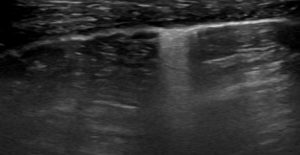
LUSS = 2 if there were vertical artifacts (B2-profile) or subpleural loss of aeration or pleural effusion associated with loss of aeration (vertical artifacts or tissue-like pattern) that involved >50% of the visualized pleural line.
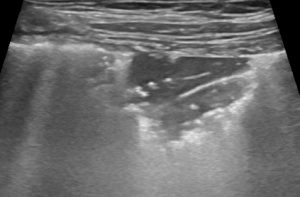
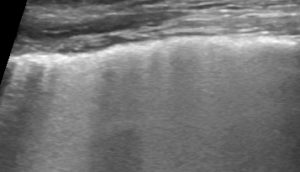

LUSS = 3 if translobar tissue-like pattern or pleural effusion occupied the entire visualized pleura without visualization of any aerated lung.
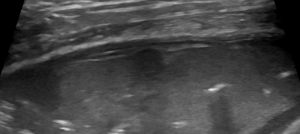
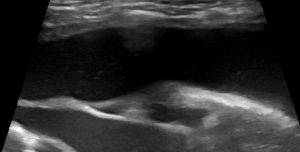
Thus the maximum cumulative score for all 8 quadrants is 24/24.
The good news is that Oricco et al. found ‘excellent reliability among all of the raters in the calculation of the LUSS as well as the identification of different patterns.’ This included a cross section of more and less experienced clinician/sonographers.
Lung ultrasound has a bit of a mixed reputation amongst different groups of clinicians (internists, criticalists, cardiologists). It’s a useful finding that one can achieve excellent reliability when the sources of potential discrepancy are eliminated by grouping together lung changes of one type. Obviously that still leaves room for further discussion of the potential causes of ‘loss of aeration’ in each individual patient -allowing for the possibility that this may be less reliable.