Lung ultrasound in cats and dogs: why does it get such a varied reception? Things become clearer
My impression, from a UK veterinary perspective, is that lung ultrasound (LUS) gets variable press. Specifically, most specialist cardiologists don’t much care for it. It’s useful to understand why this is.
It’s no coincidence that LUS was discovered and developed primarily by human acute care physicians in intensive care units (ICUs) and emergency departments. Looking at the human literature there’s no doubt that many of the best emergency and critical care clinicians use LUS all the time (in the same way that they might use a stethoscope) and find it indispensible.
J Ultrason. 2021 21(86): e225–e233.
Signs and lines in lung ultrasound
Rohit Bhoil, Ajay Ahluwalia, Rajesh Chopra, Mukesh Surya, and Sabina Bhoil
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8439137/
‘It is highly desirable to have accurate and easily reproducible imaging techniques to diagnose pathologies, and implement and monitor treatment – especially in critically ill patients. Point-of-care ultrasound (POCUS) has become firmly established in acute and critical care settings (FAST, vascular access, echocardiography), and is now increasingly being used as an important tool in the assessment of the lungs‘
Thoracic ultrasonography: a narrative review
P. H. Mayo, R. Copetti, D. Feller-Kopman, G. Mathis, E. Maury, S. Mongodi, F. Mojoli, G. Volpicelli & M. Zanobetti
Intensive Care Medicine volume 45, pages1200–1211 (2019)
https://link.springer.com/article/10.1007/s00134-019-05725-8
‘Mastery of lung and pleural ultrasonography allows the intensivist to rapidly diagnose and guide the management of a wide variety of disease processes that are common features of critical illness. Its ease of use, rapidity, repeatability, and reliability make thoracic ultrasonography the “go to” modality for imaging the lung and pleura in an efficient, cost effective, and safe manner, such that it can largely replace chest imaging in critical care practice. It is best used in conjunction with other components of critical care ultrasonography to yield a comprehensive evaluation of the critically ill patient at point of care.’
‘The utility of lung ultrasonography for identification of acute heart failure is well established to be superior to evaluation that uses CXR as the primary imaging modality‘
BMJ Open 2021 Mar 5;11(3):e045120
Development of a convolutional neural network to differentiate among the etiology of similar appearing pathological B lines on lung ultrasound: a deep learning study
Robert Arntfield 1, Blake VanBerlo 2, Thamer Alaifan 3, Nathan Phelps 4, Matthew White 3, Rushil Chaudhary 5, Jordan Ho 2, Derek Wu
https://bmjopen.bmj.com/content/11/3/e045120.citation-tools
‘Lung ultrasound (LUS) is an imaging technique deployed by clinicians at the point-of-care to aid in the diagnosis and management of acute respiratory failure. With accuracy matching or exceeding chest X-ray (CXR) for most acute respiratory illnesses, LUS additionally lacks the radiation and laborious workflow of CT. Further, as a low-cost, battery-operated modality, LUS can be delivered at large scale in any environment‘
But, at the same time, attempts to capture the utility of LUS in tightly-defined, controlled studies often yield slightly disappointing sensitivity/specificity outcomes. It’s a subtle and complicated diagnostic tool!
To quote Mayo et al.:
‘Thoracic ultrasonography has several limitations. It requires the intensivist to be competent in image acquisition, image interpretation, and the cognitive base. …. The studies referenced the present article have been performed by groups with a high-level capability in lung ultrasonography. The reader can only expect to achieve similar results, if they have a similar level of competence.‘
This is a real issue: at least in the UK, my feeling is that the narrative in cardio-thoracic imaging tends to be dominated by cardiologists and internists. Cardiology teaching in referral institutions has a long tradition of using radiography to assess lungs. These days, specialist internists often rely on CT. Naturally, one tends to trust what one knows and feels familiar with.
Broadly speaking, if one is an intensivist, critical care clinician or general practitioner faced with a patient with respiratory signs then ultrasonography offers an excellent means of efficiently narrowing down a diagnosis. On the other hand, if one is a cardiologist presented with a patient in a non-critical setting (murmur, syncope, arrhythmia) then lung ultrasound can seem a bit subjective and insufficiently reliable in diagnosing cardiogenic pulmonary oedema.
In human medicine, this observation has also been made
‘clinical context is more important for lung US than other imaging modalities.’
https://pubmed.ncbi.nlm.nih.gov/29656607/
As Mayo et al. imply, lung ultrasonography is a lot more complicated than it’s often made out to be. Why is it that ‘the reader can only expect to achieve similar results if they have a similar level of competence‘ when it’s simply a matter of B lines, A lines and consolidation?
There are issues of examination protocol and of interpretation of findings
Protocol:
With apologies to the authors of Mayo et al. above for reproducing such a chunk of their text…
‘There is no best way to perform image acquisition for thoracic ultrasonography. One elegant method utilizes three pre-determined examination points on the left hemithorax and three identical pre-determined examination points on the right hemithorax. This yields enough information to categorize the cause of acute respiratory failure in a high percentage of case. This approach is codified in the BLUE protocol. Other scanning protocols have been described in a definitive guideline document. An alternative method is for the intensivist to examine multiple adjacent rib interspaces while moving the probe in linear fashion across the thorax in a series of longitudinal scan lines. This yields a comprehensive view of the thorax. If an abnormality is detected, the operator focuses on the area of interest in more detail. There is no mandatory method of performing the thoracic ultrasonography examination. This reflects on the flexibility of the technique which is controlled by the clinician and applied according to the clinical requirements of the case.‘
Obviously, this mirrors the situation in veterinary medicine. On the one hand, there’s an understandable move to standardize and streamline protocol. On the other hand, a clinician faced with an individual case might decide that he/she simply wants to examine as much of the lung as possible in order to gather as much information as possible.
Interpretation of findings:
It cannot be stressed enough that the appearance of various features is very dependent on machine, probe and settings.
In the beginning there was no lung ultrasound. From about 2010 onwards discoveries came thick and fast: ‘classic’ patterns of LUS findings were described. Latterly, as the field has matured, we’ve realised that sometimes it gets more complicated!
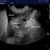
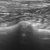
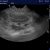
Coming to lung ultrasound with absolutely no formal training, I’ve always found that formal descriptions of various features in the (largely human) literature don’t correspond well with specific examples in real life. For example, a mainstream definition of B lines would be ‘discrete laser-like vertical hyperechoic reverberation artifacts that arise from the pleural line (previously described as ‘comet tails’), extend to the bottom of the screen without fading, and move synchronously with lung sliding‘.
Intensive Care Med 2012; 38:577–591.
International liaison committee on lung ultrasound (ILC-LUS) for the international consensus conference on lung ultrasound (ICC-LUS), international evidence-based recommendations for point-of-care lung ultrasound.
Volpicelli G, Elbarbary M, Blaivas M, et al.
Compare that with these images from the supplementary material in Mayo et al.
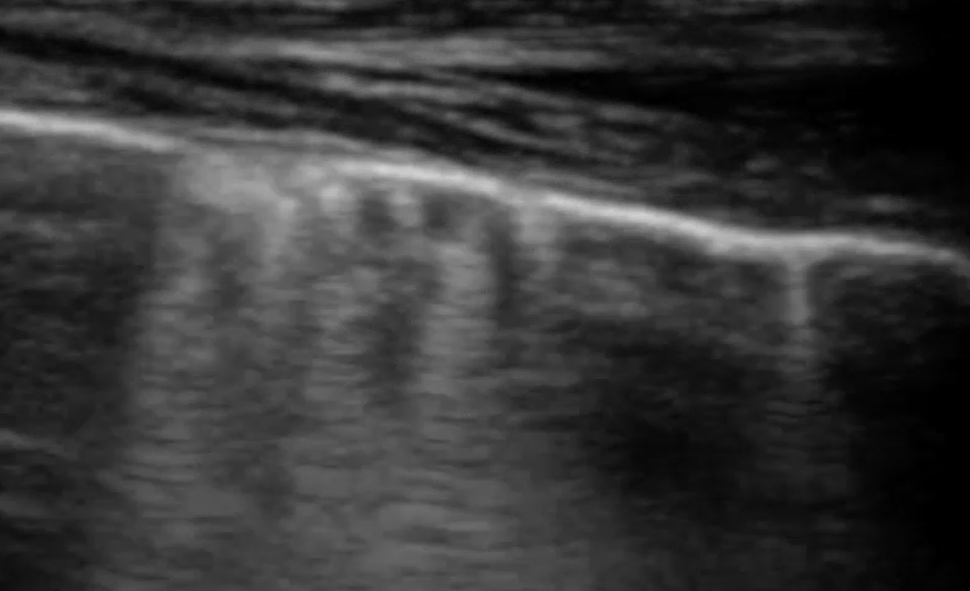
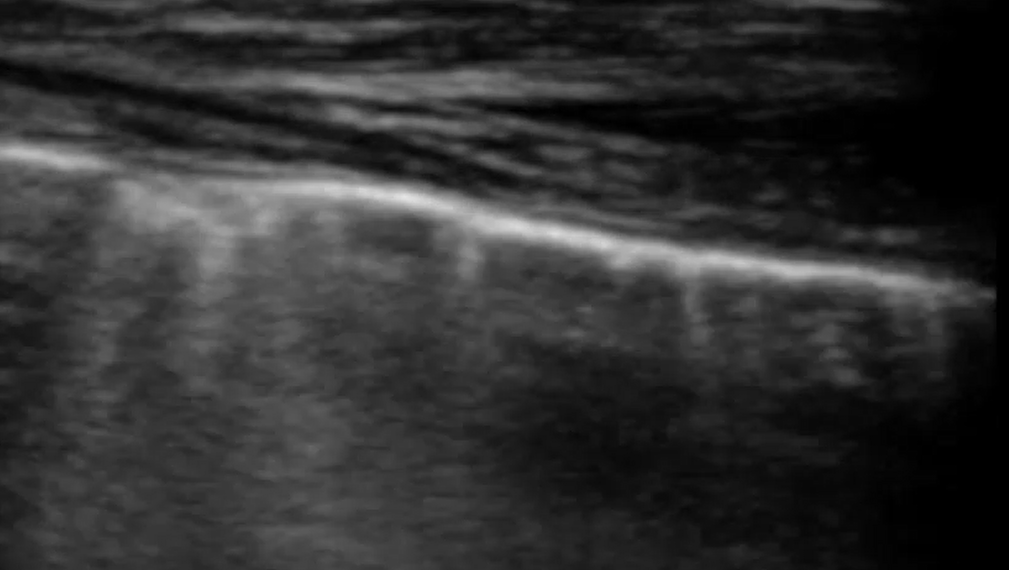
Paul Mayo is ‘a frontline intensivist in the Northwell System in the New York City area where he is academic director of critical care medicine and professor of clinical medicine at the Zucker School of Medicine at Hofstra/Northwell’. I think we have to conclude that he knows what he’s talking about and that his diagnostic conclusions reflect the consensus in human lung ultrasonography.
..and yet, I think a student coming to this armed with the mainstream definition of what constitutes a B line would be justified in thinking that the artefacts in this particular patient viewed with this particular machine/probe/setting aren’t ‘narrow based’, ‘laser like’ or ‘spreading to the edge of the screen’.
In that specific example, a high frequency linear, vascular probe is being used to optimise pleural line detail. That has a very significant effect on the appearance of the vertical lines.
Recent attempts at standardisation recognise these problems:
J Ultrasound Med 2023 Feb;42(2):309-344
New International Guidelines and Consensus on the Use of Lung Ultrasound
Libertario Demi et al.
https://onlinelibrary.wiley.com/doi/10.1002/jum.16088
‘The practical use of [the definition of B lines as stated by Volpicelli et al.] poses several challenges, and limits the accuracy and reproducibility of the associated evaluations. Many aspects of this definition are highly subjective. It is difficult to associate quantitative information with the words “laser-like” and “without fading.” Reproducible methods to evaluate the lateral extent of artifacts have not been established. It is likely that by varying the imaging depth, the artifact will no longer reach the “bottom of the screen,” and there is no defined minimum length for the vertical artifact to be referred as a B-line. Moreover, the impact of key imaging parameters (frequency, bandwidth, beam width, angle of incidence, dynamic range) on the appearance of B-lines is not accounted for in this definition.
This needs to be addressed if we are to develop a reproducible, accurate, and reliable US method dedicated to the lung. Additionally, standardization of the acquisition process is essential to minimize the effect of confounding factors.’
OK, so here, I feel we are actually getting some understanding of the problems we experience in real life! It now seems that the terms ‘i-line’ and ‘c-line’ which appear in the literature are becoming obsolete. ‘i-lines’ are just vertical artefacts at the short and less bright end of a spectrum of B-line appearance. ‘c-lines’ are B lines associated with consolidation.
To continue from Demi et al.
‘In the current definition, B-line artifacts represent a wide variety of patterns. It is crucial to understand the physical origin of their genesis and to characterize the signals responsible for their visualization. This is a fundamental step toward the development of quantitative US modalities dedicated to the diagnosis and monitoring of lung diseases.
Guidelines:
In order to guarantee the reproducibility of LUS studies, always report explicitly the adopted MI range, the probe and scanner utilized, the imaging frequency range, the focal and imaging depth range as well as the areas of the chest examined, chest wall thickness and the rationale for the choices made. LUS image analysis could include the investigation of pleural effusion (not an artifact), characterization of the pleural line (not an artifact) and consolidations (not an artifact), as well as, the study of vertical artifacts (eg, B-lines, white lung), horizontal artifacts (A-lines), and pleural motion. Acknowledge that the appearance of LUS artifacts is operator dependent. Refer to B-line counting as a semi-quantitative method as that is not an absolute measure but a relative and operator-dependent measure. To the same extent, any qualitative characterization of LUS artifacts concerning their length, image intensity and appearance should always acknowledge the impact of the utilized hardware and imaging settings on these properties.’
The language of lung ultrasound has some way to go. And the language is really important. It’s no good quantifying B lines and doing a lot of stats on the outcomes if one can’t consistently say what a B line is!
Similar problems apply to pleural line assessment:
‘Currently, ….analysis of the pleural-line and subpleural space is mostly limited to a qualitative, subjective, not measurable or reproducible evaluation. There is a lack of evidence in the scientific literature regarding a clear definition and measure of regularity/irregularity/thickening/fragmentation of the pleural line or of the dimensional and quantitative distinction between subpleural micro- and macro-consolidations.‘
That’s another quote from Demi et al.
An illuminating recent study:
BMJ Open 2021 Mar 5;11(3):e045120
Development of a convolutional neural network to differentiate among the etiology of similar appearing pathological B lines on lung ultrasound: a deep learning study
Robert Arntfield 1, Blake VanBerlo 2, Thamer Alaifan 3, Nathan Phelps 4, Matthew White 3, Rushil Chaudhary 5, Jordan Ho 2, Derek Wu
https://bmjopen.bmj.com/content/11/3/e045120.citation-tools
..shows that AI ‘can distinguish similar appearing LUS pathology…that cannot be distinguished by humans‘. When freed from constraints of language, there’s more information to be extracted from lung ultrasound images than we’re currently getting. I suspect that this is why really experienced lung sonographers achieve different outcomes compared to other clinicians…but struggle to explain exactly why.
As the authors of Arntfield et al. say: ‘user-dependent interpretation of LUS contributes to wide variation in disease classification‘.
Not only are there issues with settings and definitions. Some clinical scenarios involve complicated overlap between the ‘classic’ patterns of LUS findings.
J Ultrasound Med. 2018 Nov;37(11):2659-2665
Expert Agreement in the Interpretation of Lung Ultrasound Studies Performed on Mechanically Ventilated Patients
Scott J Millington 1, Robert T Arntfield 1, Robert Jie Guo 1, Seth Koenig 1, Pierre Kory 1, Vicki Noble 1, Haney Mallemat 1, Jordan R Schoenherr
https://pubmed.ncbi.nlm.nih.gov/29656607/
‘The sensitivity and specificity of the discrete, well-defined lung US patterns have been derived by comparison to a reference standard such as computed tomography, usually in patients presenting
to the emergency department with a single acute problem (typically dyspnea). Our cohort involved scans performed primarily on mechanically ventilated ICU patients with varying lengths of stay in the ICU, who
were therefore much more likely to have multiple coexisting lung insults as well as chronic lung diseases.‘
and they found…
‘The rate of agreement between experts….was …lower than might have been expected; this finding was
likely driven by several factors. Chief among the potential explanations was the coexistence of multiple abnormal lung US patterns in many patients, making it challenging to agree on which was the primary problem. The most common areas of disagreement centered on deciding on the relative importance of consolidative-like patterns; the 3 specific disagreements that occurred most commonly all involved possible lung consolidation. This finding was, perhaps, understandable given the well-known difficulties with each diagnostic pairing. An atelectatic lung, for example, can be difficult to distinguish from consolidation, which typically represents pneumonia. Determining the relationship between pleural effusion and a consolidated lung is also challenging, in which pneumonia can be the main problem (with the effusions representing as para-pneumonic process), or the effusion can be the primary issue (with the consolidation being, in fact, associated compressive atelectasis). Distinguishing consolidation from interstitial syndrome can also be difficult, as both can generate areas of B-lines on lung US images.‘
‘In conclusion, the ability of experts to interpret lung US studies of variable quality in the absence of clinical information for complex ICU patients may be lower than expected. This finding should inform the manner in which such studies are interpreted in clinical practice, arguing for a cautious approach and the inclusion of as much clinical information as possible. Particular care should be exercised when cases involve potential consolidation patterns.‘
It’s worth saying here that, personally, I am dispensing with the use of the terms ‘shred sign’ and ‘fractal sign’ which I find aren’t intuitively understood by most students coming to LUS. ‘Non-translobar consolidation’ is less catchy but, I think, more straightforward.