Lung ultrasound: contusions, interstitial syndrome, A, B, C, E, I and Z-lines explored
I reckon lung ultrasound must be one of the most profound developments in veterinary medicine during my career. The facility to diagnose rapidly, and with high degree of confidence, pneumonia, pulmonary oedema, contusions, pneumothorax and neoplasia is a phenomenal advance. Having arrived in our tool-kit in the last 10 years, this is still an evolving field. In this post I want to look at the classification of lung lines and their significance.
Normal transthoracic lung ultrasound reveals a pattern of repetetive artifactual ‘A lines‘ all parallel to the pleura. These are the result of sound bouncing back and forth between probe head, pleura and tissue planes in the thoracic wall.
Comet-tail artifacts is the generic term for lines which run perpendicular to the pleura: subgroups include B lines, C lines, E-lines, I lines and Z lines. These all arise from short-path reverberations within confined spaces.
The term ‘interstitial syndrome’ (IS) describes the sonographic appearance of lung in which there is increased extra-vascular water content (oedema) interspersed with air-filled alveoli. This provides the conditions for reverberation which causes B lines which are the hallmark of the syndrome. B lines arise at the pleura, run down to the limit of the image, move with the respiratory movements of the lung and overlie the normal A lines. This much we have understood for a while.
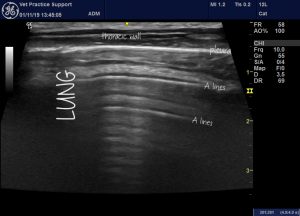
Normal lung: transverse plane transthoracic lung ultrasound in a dog
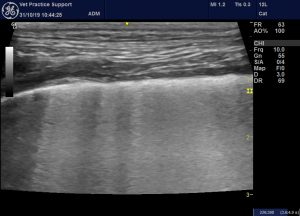
Interstitial syndrome: multiple B lines in the lung of a dog. B lines arise at the pleura run to the bottom of the image, obscure the normal A lines and move with respiratory movements.
A wide spectrum of pathologies may cause IS: from left-sided congestive heart failure, overhydration, trauma, pneumonitis, interstitial fibrosis, near-drowning, acute respiratory distress syndrome, lung lobe torsion, electrocution to drug reactions and disseminated neoplasia.
J Ultrasound Med. 2009 Feb;28(2):163-74.
Sonographic interstitial syndrome: the sound of lung water.
Soldati G1, Copetti R, Sher S.
https://onlinelibrary.wiley.com/doi/full/10.7863/jum.2009.28.2.163?sid=nlm%3Apubmed
As time has gone by other subgroups of ‘comet-tail’ artifact have been described:
I lines are short lines arising at the pleura which, unlike B lines, fade as they run deeper. They also move with the lung on respiration. It is important to note that these become much more apparent when high-frequency probes are used. I tend to prefer using a 10 MHz linear probe and, with this, they are much more commonly apparent than with a microconvex probe.
Some I lines may be seen in ‘normal’ lung, especially with a high frequency probe and towards the margins of lobes. Hence, they are not as reliably indicative of pathology as B lines. However, there is a tendency for them to become more prominent with increasing fibrosis or inflammation. My impression is that they are commoner in older animals and in cats more than in dogs.
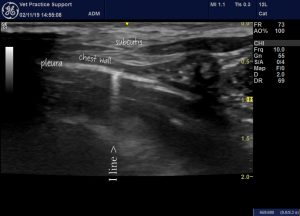
An ‘I line’ in the lung of a healthy 6-month-old dog. These are especially common towards the margins of lobes.
As described and discussed in human medicine here:
J Intensive Care. 2016; 4(1): 57.
Lung ultrasound—a primary survey of the acutely dyspneic patient
Francis Chun, Yue Lee
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5007698/
C lines are broad ‘hyperechoic’, ‘stripes which run perpendicular to the pleura and originate from a thickened, often irregular, hypoechoic pleural line. In human medicine these are thought to be highly specific for pneumonia in cases without parenchymal consolidation.
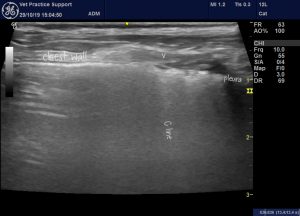
C lines in the lung of a 4 y.o. Dachshund presenting with an acute, febrile illness, tachypnoea and neutrophilia. Pneumonia is the usual cause of C lines in people.
This appearance is more subtle than the classic ‘shred’ sign of bronchopneumonia.
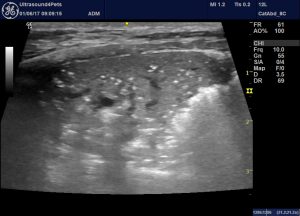
Classic appearance of bronchopneumonia in a dog: consolidated lung with air bronchiolograms, serrated margins and hyperechoic periphery with comet-tail artifacts. This is the ‘shred sign’ or ‘fractal sign’.
Examples of the phenomenon from human medicine here:
Z lines, to quote Chun and Lee (cited above)…
‘Z-lines (reverberation artifacts) These artifacts could be randomly found in any part of the lungs during LUS exam and are likely to be caused by short-paths reverberations between the parietal pleural and the endothoracic fascia. Because of the extra-pulmonary location, they are often seen as static vertical artifacts which do not move with lung sliding. Z-lines do not have any clinical significance except that they could easily be misinterpreted as B-lines‘
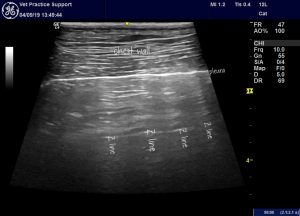
Z lines in a dog: these hyperechoic stripes don’t move with the lung during respiratory movements. They are usually relatively faint comapred to ‘laser-like’ B lines.
E-lines are generated by subcutaneous emphysema; they are vertical laser-like lines that reach the edge of the screen but do not arise from the pleural line.
Peripheral parenchymal lesion (PPL):
Ultrasound has an important role in point-of-care diagnosis (obviously, dyspnoeic cats and dogs with lung contusions don’t always present with a history of known trauma) and staging of lung trauma.
Chest. 2012 May;141(5):1177-1183. doi: 10.1378/chest.11-0208. Epub 2011 Oct 20.
Diagnostic accuracy of ultrasonography in the acute assessment of common thoracic lesions after trauma.
Hyacinthe AC1, Broux C1, Francony G1, Genty C2, Bouzat P1, Jacquot C1, Albaladejo P1, Ferretti GR3, Bosson JL4, Payen JF1.
These authors describe the appearance of lung contusions as:
‘(1) an irregularly delineated tissue image, which could be a moderately hypoechoic blurred lesion with no change during respiration or hyperechoic punctiform images corresponding to air bronchogram;
and/or
(2) multiple B-lines‘
Similarly,
Emergency (Tehran). 2018; 6(1): e55
Screening performance of Ultrasonographic B-lines in Detection of Lung Contusion following Blunt Trauma; a Diagnostic Accuracy Study
Saeed Abbasi,1 Hossein Shaker,1 Fariba Zareiee,1 Davood Farsi,1 Peyman Hafezimoghadam,1 Mahdi Rezai,1 Babak Mahshidfar,1 and Mani Mofidi1,*
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6289153/
…also describe two lesion types:
‘a) Alveolo-interstitial syndrome (AIS) by the presence of multiple B-lines (more than 3) that originate from pleural line in a person with no clinical cardiopulmonary signs (figure 1-B).
b) Peripheral parenchymal lesion (PPL), defined by the presence of C-lines: hypoechoic subpleural focal images with or without pleural line gap‘
Elsewhere, peripheral parenchymal lesion is ‘defined as the observation of C-lines, confluent consolidations (“hepatization”), or the presence of parenchymal disruption with localized pleural effusion‘
D. Lichtenstein, G. Mezière
The BLUE-points: three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure
Crit. Ultrasound J., 156 (2011), pp. 1640-1646
https://theultrasoundjournal.springeropen.com/articles/10.1007/s13089-011-0066-3
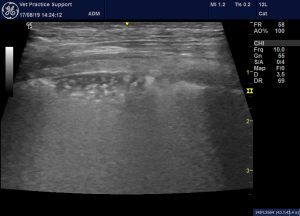
And that is entirely consistent with what we see in dogs and cats. This is a previously healthy dog with a history of a fall from about 1 metre about 2 hours prior to lung ultrasound:
The area of PPL as a frozen image:
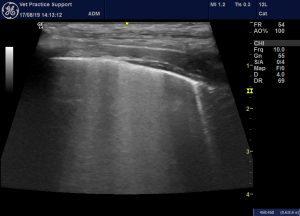
…and surrounding that, B lines:
Egyptian Journal of Chest Diseases and Tuberculosis
Volume 64, Issue 2, April 2015, Pages 469-475
Role of chest ultrasonography in the diagnosis of lung contusion
ShadiaHelmyaBassemBeshaybMohamedAbdel HadybAbdelmenamMansour
https://www.sciencedirect.com/science/article/pii/S0422763814200719#b0025
…reported ‘sensitivity of lung ultrasonography in detection of lung contusion [in human patients] was 97.50%, specificity was 90.0%’
Where CT was used as gold standard for comparison.
That’s very similar to:
Chest. 2006 Aug;130(2):533-8.
Chest ultrasonography in lung contusion.
Soldati G1, Testa A, Silva FR, Carbone L, Portale G, Silveri NG.
https://www.ncbi.nlm.nih.gov/pubmed/16899855
Who reported….’the sensitivity of ultrasound study was 94.6%, specificity was 96.1%‘ compared to ‘Radiography with sensitivity of 27% and specificity of 100%‘
PPL has the same qualitative appearance as lung torsion. The difference being that contusions don’t usually correlate exactly with the limits of one lobe.
The evolution of lung contusions and possible complications in man are described by Bassem et al. thus:
‘After the initial blunt or blast thoracic trauma, the edematous phase is notable for worsening interstitial edema and infiltrates, occurring within the first 1–2 h after injury. The air spaces become inundated with blood, inflammatory markers, and tissue debris, as there is an increase in alveolar and capillary permeability along with a reduction in surfactant production. Within 24–48 h after the onset of injury, there is alveolar collapse and further consolidation due to the extravasation of blood into the alveoli. Lung consolidation can lead to increased vascular pressures causing pulmonary hypertension and retention of blood. The resulting ventilation/perfusion mismatch, increased pulmonary shunting, decreased gas exchange, and decreased compliance can predispose patients to clinically apparent symptoms such as hypoxia, hypercarbia, tachypnea, hemoptysis, and wheezing. It is also these mechanisms of consolidation, shunting, and mismatch that predispose patients with pulmonary contusions to pneumonia and acute respiratory distress syndrome (ARDS)‘

Fantastic article Roger!
Second that, very interesting details and excellent images
Thanks Roger