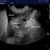
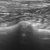
Is this dog with heart disease in congestive failure?
The more you know the more you realise how little you know. On the face of it, the principle of diagnosing congestive heart failure (CHF) is simple enough. Until you start seeing cases where, in practice, it’s not simple at all! It’s reassuring to find that human medics have the same problem.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10375124/
‘Diagnosing non‐secondary heart failure (HF) … can be a challenge, especially in normovolaemic patients without a prior hospitalization for HF. Accordingly, algorithms and scores have been proposed in recent years, to help clinicians evaluating patients with exertional dyspnoea‘
Algorithms and scores…. this says it all. We know already that it’s not going to be a matter of measuring a couple of things on an echocardiogram. Response to diuretic treatment is fair enough: but I’d hope we aspire to something more prospective.
As we’ve discussed in a previous blog post; one’s take on all of this depends very much on one’s patient population. For a cardiologist with a caseload including a high proportion of patients presenting with evidence of heart disease (a murmur, for example) this pre-selection tends to filter out a lot of animals with other medical issues. In that population the diagnostic focus is on distinguishing between patients with early heart disease and those with CHF. For an internist faced with a caseload of patients with, for example, respiratory signs or ascites then the emphasis is on distinguishing between animals with CHF and those with other diseases (or both).
It’s one thing to say whether a patient has heart disease or not. The crunch issue often remains ‘is this animal in congestive failure’ -because that’s usually key to the matter of whether any clinical signs are attributable to cardiac disease and will be central to treatment issues.
So, lets go to a couple of recent veterinary papers for guidance:
J Vet Intern Med. 2019 May-Jun; 33(3): 1127–1140.
ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs
Bruce W. Keene 1 Clarke E. Atkins, 1 John D. Bonagura, 1 , 2 Philip R. Fox, 3 Jens Häggström, 4 Virginia Luis Fuentes, 5 Mark A. Oyama, 6 John E. Rush, 7 Rebecca Stepien, 8 and Masami Uechi
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6524084/
‘Recommendations for diagnosis of Stage C:
The signalment, history, and physical examination can be helpful in determining the pretest probability of heart failure as a cause of clinical signs in patients with MMVD. For example, …dogs with marked sinus arrhythmia and relatively slow heart rates also are less likely to have clinical signs attributable to MMVD than are those with similar clinical signs (eg, cough, dyspnea) with sinus rhythm or sinus tachycardia. (Class I, LOE: expert opinion)’
The typical dog in Stage C from MMVD presents with clinical signs of left‐sided CHF and a history that can include tachypnea, restlessness, respiratory distress, or cough. A clinical database (including thoracic radiographs and ideally an echocardiogram) should be obtained.
Echocardiography with Doppler studies also is useful in the diagnosis of dogs with MMVD that have advanced to Stages C and D. Cardiac ultrasound examination can confirm the presence of MMVD, quantify chamber enlargements and cardiac function, provide general estimates of LV filling pressures, and identify comorbidities and complications of chronic MR. As an example, a pretreatment finding of a low‐velocity E‐wave on pulsed‐wave Doppler strongly argues against a diagnosis of left‐sided heart failure. Conversely, most dogs in Stages C and D have high‐velocity early filling waves. In dogs with evidence of symptomatic pulmonary hypertension (eg, exertional fatigue, collapse or syncope, ascites from right‐sided CHF), spectral Doppler findings can substantiate the diagnosis and help guide therapeutic decision‐making.’
Serum NT‐proBNP concentrations (obtained using a commercially available test) can add useful adjunct evidence when determining the cause of clinical signs in dogs with MMVD, especially when the NT‐proBNP concentration is normal or nearly normal in a symptomatic animal. …. A normal or near normal NT‐proBNP concentration in a dog with clinical signs of cough, dyspnea, or exercise intolerance strongly suggests that heart failure is not the cause of the clinical signs‘
And, bang-up-to-date:
J Vet Intern Med 2023 Sep 11
Radiographic lung congestion scores in dogs with acute congestive heart failure caused by myxomatous mitral valve disease
Liza Koster 1, Jenna Vogel 1, Cary M Springer 2, Silke Hecht 1
https://onlinelibrary.wiley.com/doi/full/10.1111/jvim.16850
‘The diagnosis of acute CHF in a dog with myxomatous mitral valve disease (MMVD) is a clinical one, made considering the constellation of a typical signalment, a presenting complaint of tachypnea, the presence of a moderate to high-intensity murmur consistent with severe mitral insufficiency, left atrial enlargement on cardiac imaging, lung infiltrates, and the rapid resolution of clinical signs (and lung infiltrates) in response to furosemide administration‘
At least everyone seems to be in agreement that a diagnosis of CHF relies on consideration of various parameters.
As a non-specialist sonographer/medic, my first comment on the ACVIM consensus statement is that this is, unsurprisingly, the cardiologist perspective. Heart rate, respiratory rate, changes in pro-BNP, echocardiographic evidence of mitral valve disease…. this is all well and good but if the dog you’re looking at might have primary lung disease plus heart disease then it’s not much help.
Heart rate:
The authors of the consensus statement assign the assertion that heart rate is useful to ‘expert opinion’. But there is actually some evidence on these issues to be examined. Comparison of heart rates between dogs (undergoing echocardiography) in CHF and those in earlier stages of heart disease is available from the seminal:
J Vet Intern Med 2010 Nov-Dec;24(6):1358-68
Detection of congestive heart failure in dogs by Doppler echocardiography
K E Schober 1, T M Hart, J A Stern, X Li, V F Samii, L J Zekas, B A Scansen, J D Bonagura
https://onlinelibrary.wiley.com/doi/10.1111/j.1939-1676.2010.0592.x
From table 1:
| median heart rate | 5/95 percentiles | |
| MVD with CHF | 140 | 100-206 |
| MVD without CHF | 132 | 91-169 |
| Primary myocardial failure ‘DCM’ with CHF | 160 | 120-210 |
| Primary myocardial failure ‘DCM’ without CHF | 114 | 70-198 |
Equivalent data can be gleaned from:
Vet J 2020 Sept:263:105518
Echocardiographic parameters for the assessment of congestive heart failure in dogs with myxomatous mitral valve disease and moderate to severe mitral regurgitation
K R S Morgan 1, G Monteith 1, S Raheb 1, M Colpitts 1, S Fonfara 2
https://pubmed.ncbi.nlm.nih.gov/32928487/
…which is, in many ways, a re-visitation of the same subject matter a decade on.
| mean heart rate | SD | |
| MVD with CHF | 145 | 21 |
| MVD without CHF | 130 | 21 |
So that’s a decent degree of similarity between the two studies.
If one looks at Holter-derived mean heart rates from MVD dogs:
Vet Rec 2012 Jun 16;170(24):622
Heart rate variability parameters of myxomatous mitral valve disease in dogs with and without heart failure obtained using 24-hour Holter electrocardiography
M S Oliveira 1, R A L Muzzi, R B Araújo, L A L Muzzi, D F Ferreira, R Nogueira, E F Silva
https://pubmed.ncbi.nlm.nih.gov/22645158/
| mean heart rate | SD | |
| MVD with CHF | 125 | 19 |
| MVD without CHF | 97 | 17 |
They’re, unsurprisingly, a bit lower but still in the same ballpark.
I’d add that my personal observation is that if one performs echocardiography in standing dogs their heart rate is 10-20 beats/minute lower than if they are restrained in lateral recumbency.
So, overall, it is true that dogs with CHF have slightly higher heart rates; but the high degree of overlap in MVD dogs means that it’s probably not a lot of use in practice. For dogs with ‘DCM’ there is some evidence that those with heart rates <120 may be less likely in CHF. One needs in factor in the circumstances of the examination when judging heart rate.
For cats, heart rate is probably even less reliable since we know that some cats with CHF are relatively bradycardic.
https://pubmed.ncbi.nlm.nih.gov/1517140/
The consensus statement suggests that presenting signs and presence of murmur aren’t reliable evidence of CHF -which we can all agree with. There is mention of thoracic radiographs but no specific examination of any specific criteria or diagnostic accuracy. Again, it doesn’t leave us much the wiser. There’s absolutely no mention of lung ultrasound (LUS) in either article! Published in 2019 and 2023 that does seem a bit of an oversight.
The diagnostic utility of thoracic radiography (CXR) in congestive heart failure is worth a critical look. Obviously, for decades the convention has been that CXR is fundamental to confirming cardiogenic pulmonary oedema. For example (from a team of eminent cardiology diplomates in 2021 ):
https://cardiaceducationgroup.org/wp-content/uploads/2021/05/CEG-ABCD-Brochure_043021-1.pdf
‘Point of care ultrasound to identify pulmonary infiltrates and cavitary effusions may be helpful but are not a substitute for thoracic radiographs to diagnose the presence of congestive heart failure‘
To be brutally honest, I’ve never liked that dictum because I find CXR unreliable in my hands: I’m happy to find support in the literature:
https://onlinelibrary.wiley.com/doi/full/10.1111/jvim.16850
‘Distinguishing CPE from other causes of abnormal lung patterns on thoracic radiographs is challenging‘
Sci Rep 2021 Feb 17;11(1):3964
Automatic classification of canine thoracic radiographs using deep learning
Tommaso Banzato 1, Marek Wodzinski 2, Silvia Burti 3, Valentina Longhin Osti 3, Valentina Rossoni 3, Manfredo Atzori 4 5, Alessandro Zotti
https://www.nature.com/articles/s41598-021-83515-3
‘In human medicine, despite the efforts to improve radiology residents’ training programmes, the prevalence of interpretation errors has not significantly improved in recent decades. The prevalence and the impact of interpretation errors on thoracic radiographs have only seldom been investigated in veterinary medicine. Conversely, this topic has been widely studied in human medicine and the most common causes of interpretation errors have been identified‘
In human medicine, the evidence suggests that CXR is not very accurate in the diagnosis of cardiogenic pulmonary oedema/edema (CPO/CPE) and, specifically, not as accurate as lung ultrasound.
JAMA 2019 Mar 1;2(3):e190703
Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis
Anna M Maw 1, Ahmed Hassanin 1, P Michael Ho 2 3, Matthew D F McInnes 4 5, Angela Moss 6, Elizabeth Juarez-Colunga 6 7, Nilam J Soni 8 9, Marcelo H Miglioranza 10, Elke Platz 11 12, Kristen DeSanto 13, Anthony P Sertich 14, Gerald Salame 1, Stacie L Daugherty
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6484641/
Sensitivity of LUS was 0.88 and specificity 0.90. For CXR the equivalent figures were 0.73 and 0.90.
Obviously, lung ultrasound is highly dependent on operator and machine/probe/settings factors. Among the studies included in this meta-analysis the sensitivity of LUS varied widely from 0.58 – 097. This might be due to different individual thresholds and criteria for positivity. For example, there’s some heterogeneity as to what constitutes a ‘B line’.
Am J Cardiol 2022 Jul 1:174:89-95
Meta-Analysis of Point-of-Care Lung Ultrasonography Versus Chest Radiography in Adults With Symptoms of Acute Decompensated Heart Failure
Leonard Chiu 1, Meghan P Jairam 2, Ronald Chow 3, Nicholas Chiu 4, Max Shen 4, Adam Alhassan 5, Chun-Han Lo 6, Austin Chen 7, Peter J Kennel 8, Timothy J Poterucha 8, Veli K Topkara
https://pubmed.ncbi.nlm.nih.gov/35504747/
Another good meta-analysis from human medicine with the same outcome: LUS has better sensitivity and, in this case, slightly better specificity too. Numbers are reassuringly similar to the previous paper.
As Banzato et al. say: ‘The interpretation of thoracic radiographs is a challenging and error-prone task for veterinarians.‘
In small animal medicine we don’t have a whole lot of evidence on which to judge the matter of diagnostic accuracy of CXR for CHF. We do have some studies looking at the performance of LUS:
J Vet Intern Med. 2017 May-Jun; 31(3): 700–704.
Assessment of Lung Ultrasound B‐Lines in Dogs with Different Stages of Chronic Valvular Heart Disease
T. Vezzosi,corresponding author 1 T. Mannucci, 1 A. Pistoresi, 1 F. Toma, 1 R. Tognetti, 1 E. Zini, 2 , 3 , 4 O. Domenech, 2 E. Auriemma, 2 and S. Citi 1
‘Our findings indicate that LUS has good diagnostic accuracy in identifying cardiogenic PE’
…but, since CXR was a reference tool in this study, it’s difficult to draw many conclusions about the merits of LUS as an independent predictor. One might have some doubts about the categorisation of some individual dogs in this series given that transmitral E wave Vmax was reportedly as low as 0.66 in ‘stage C’ dogs. This is not so much a criticism as an observation that it’s difficult to create a reference ‘gold standard’ groups of ‘CHF’ or ‘not CHF’ dogs with which to compare. Thus, when the authors report ‘lung ultrasound examination had a 90.0% sensitivity and 93.0% specificity in differentiating dogs with or without PE’, these numbers are in reality a reflection of the merits and limits of both CXR and LUS (as the authors themselves freely acknowledge….and I really like their study).
Another recent case series:
J Vet Intern Med. 2021 Jan-Feb; 35(1): 68–77.
Utility of point‐of‐care lung ultrasound for monitoring cardiogenic pulmonary edema in dogs
Shane D. Murphy, 1 Jessica L. Ward,corresponding author 1 Austin K. Viall, 2 Melissa A. Tropf, 1 Rebecca L. Walton, 1 Jennifer L. Fowler, 1 , 4 Wendy A. Ware, 1 and Teresa C. DeFrancesco
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7848339/
‘Overall, the results of our study suggest that B‐line artifacts improve quickly and track with TXR and clinician‐assessed clinical improvement (or relapse) of pulmonary edema in dogs with L‐CHF.’
This study doesn’t add many hard statistics on the diagnostic accuracy of LUS: but it presents a large body of data showing a reassuringly strong empirical correlation between LUS findings, CXR, respiratory rate in dogs under treatment for CHF.
There’s a great pair of studies from largely the same team of authors:
J Am Vet Med Assoc 2019 Sep 1;255(5):574-583
Lung ultrasonography findings in dogs with various underlying causes of cough
Jessica L Ward, Gregory R Lisciandro, Wendy A Ware, Kristina G Miles, Austin K Viall, Teresa C DeFrancesco
https://avmajournals.avma.org/view/journals/javma/255/5/javma.255.5.574.xml
Accuracy of point-of-care lung ultrasonography for the diagnosis of cardiogenic pulmonary edema in dogs and cats with acute dyspnoea
Jessica L Ward, Gregory R Lisciandro, Bruce W Keene, Sandra P Tou, Teresa C DeFrancesco
J Am Vet Med Assoc 2017 Mar 15;250(6):666-675
In both, the ultimate reference point was the final diagnosis on a case by case basis after review; considering all test results, response to treatment and, sometimes, post-mortem. This adds to their value I think.
These authors make the useful observation that, while presence/absence of alveolar-interstitial syndrome on LUS (B lines) are obviously important, when looking at a population of dogs with either cough or dyspnoea, factoring in the presence/absence of lung consolidation further adds to diagnostic accuracy. For coughing dogs, the finding of increased B lines (using specified scan protocol and cut offs) and absence of lung consolidation was associated with sensitivity of 92% and specificity of 99% for cardiogenic pulmonary oedema. For acutely dyspnoeic dogs, they argue, the specificity of lung ultrasound will be lower because that patient subset includes animals with non-cardiogenic alveolar-interstitial syndrome which share the LUS appearance of CPO such acute respiratory distress syndrome (ARDS), pulmonary thromboembolism and diffuse neoplasia.
From a practical point of view, it’s obviously a lot easier, quicker, safer and more convenient to use lung ultrasound…especially in veterinary patients. This becomes even more important if one is looking to perform serial assessments in an individual patient.
To avoid writing a whole book on lung ultrasound, I also want to talk about echocardiography.
The main concept here is the use of Doppler to assess transmitral flow (especially peak early diastolic [E wave] flow velocity) as an indicator of LV filling pressure (most commonly driven by increased left atrial pressure). With or without factoring in isovolumic relaxation time (IVRT) which provides some correction for the potential effect of impaired relaxation.
For a long time, the gold standard was the 2010 paper of Schober et al. quoted above. And those authors found that:
‘The major finding is that E:IVRT, Diastolic Functional Class, and IVRT allow for a rapid and feasible estimation of whether or not CHF is present. In addition, respiration rate taken at physical exam was equally useful in the prediction of CHF. Moreover, disease-specific differences between dogs with MVD or DCM regarding the diagnostic accuracy of individual DE variables and clinical decision thresholds were identified and thus need to be considered clinically‘
Obviously, respiratory rate alone isn’t a useful discriminatory parameter unless one is dealing with a set of patients in which heart disease is the only consideration.
In most clinical situations the take home message was that E:IVRT was the best Doppler-derived predictor of L CHF without having to resort to tissue Doppler (which can be required to distinguish diastolic functional class) with an optimal cut off of 1.9 for ‘DCM’ and 2.5 for MVD. In this analysis, optimal E wave velocity cut off was calculated at 1.1 m/s and at that level the predictive value of E wave alone was inferior to E:IVRT.
So that was the gospel according to Schober et al. until ….
Vet J 2020 Sep:263:105518
Echocardiographic parameters for the assessment of congestive heart failure in dogs with myxomatous mitral valve disease and moderate to severe mitral regurgitation
K R S Morgan 1, G Monteith 1, S Raheb 1, M Colpitts 1, S Fonfara 2
https://pubmed.ncbi.nlm.nih.gov/32928487/
‘The echocardiographic variable E:IVRT was significantly higher in MMVD with CHF, as previously reported (Schober et al., 2008b, 2010) but was not found to be predictive. The mean E:IVRT determined by the current study was similar to a previously reported value for dogs with MMVD without CHF (1.76 in the present study vs 1.74), but not for dogs with MMVD and CHF (2.28 in the present study vs. 3.71; Schober et al., 2010). This difference is attributed to a shorter IVRT in the CHF group of Schober et al. (2010), which measured a median of 36 ms, compared to our study, which reports a mean of 67 ms (median, 65 ms).’
Personally-speaking, my first thought on reading that was ‘Aha!, I’m not the only one who finds that IVRT measurements are technically difficult’. It’s certainly changed my outlook on E:IVRT as a tool.
There’s a lot to say about tissue Doppler and diastolic function which I shall have to come back to in another post.